
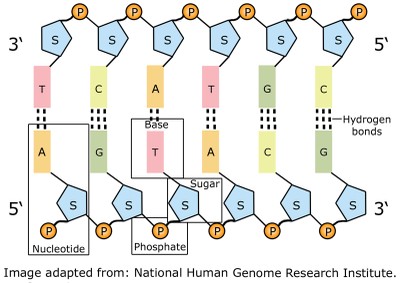
Ramachandran ( Gopalasamudram Narayan Ramachandran). This conformation is used merely as a reference point for describing the angles of rotation.Īllowed values for Φ and ψ are graphically revealed when ψ are plotted versus Φ in a Ramachandran plot, introduced by G. The conformation in which both Φ and ψ are 0 0 is prohibited for this reason. In principle, Φ and ψ can have any value between +180 and -180 0, but many values are prohibited by steric interference between atoms in the polypeptide backbone and amino acid side chains. DNA normally exists as a two antiparallel complementary strands held together by hydrogen bonds between adenines (A) and thymines (T), and between guanines (G) and cytosines (C). Figure 3: All polynucleotides contain an alternating sugar-phosphate backbone. Deoxyribonucleic acid is a polymer chain of nucleotides connected by 5’ to 3’ phosphodiester bonds. Again, by convention, both Φ and ψ are defined as 180 0 when the polypeptide is in its fully extended conformation and all peptide groups are in the same plane. It is this alternating sugar-phosphate arrangement that forms the 'backbone' of a DNA molecule.
Protein backbone versus dna how to#
The six atoms of the peptide group lie in a single plane, with the oxygen atom of the carbonyl group and the hydrogen atom of the amide nitrogen Trans to each other.įrom these findings, Pauling and Corey concluded that the peptide C-N bonds are unable to rotate freely because of their Partial double-bond character.
The oxygen has a partial negative charge and the nitrogen has a partial positive charge, setting up a small electric dipole.


 0 kommentar(er)
0 kommentar(er)
